 PDF(3387 KB)
PDF(3387 KB)


Response of nodulation and nitrogen fixation and grain yield and quality of high oil soybean to nitrogen fertilizer
Shi ZOU, Jun YAN, Rui-min GAO, Wen-xiu ZOU, Xin-chun LU, Xu CHEN
CHINESE JOURNAL OF OIL CROP SCIENCES ›› 2023, Vol. 45 ›› Issue (4) : 836-844.
 PDF(3387 KB)
PDF(3387 KB)
 PDF(3387 KB)
PDF(3387 KB)
Response of nodulation and nitrogen fixation and grain yield and quality of high oil soybean to nitrogen fertilizer
A reasonable nitrogen (N) application method could improve nodulation and N2 fixation, and reduce agricultural pollution and improve production efficiency under the premise of high yield and quality of soybeans. The effects of different N application rate and time on biomass, N2 fixation, ureide, yield, fat and isoflavone content of soybean, and the relationships among them were studied in present study, then to provide a theoretical and scientific basis for high-yield and high-quality soybean in this area. A pot experiment was conducted to study the effects of N application rates with 0 (CK), 5 mg N/kg soil (LN) and 100 mg N/kg soil (HN) at V2 (two trifoliolates) or R1 (beginning bloom) stages on growth, nitrogenase activity and ureide content at R2 (full bloom) and R5 (beginning seed) stages. The yield, fat and isoflavone of soybean seeds were determined at R8 (full maturity) as well. The N application rate and time had significant effects on nodulation and nitrogenase activity of soybean. With increasing N application rate, the nodule dry weight and number were decreased. Compared with the CK treatment, the nodule number and dry weight decreased by 69.6% and 88.4% under HN with R1 treatment at the R2 stage, respectively. In contrast, the highest nitrogenase activity and ureide content were observed under LN treatment. Compared to the CK treatment, the nitrogenase activity under LN with V2 treatment increased by 28.5% and 18.2% at the R2 and R5 stage. Soybean grain yield and fat content were the highest in LN treatment, while isoflavone content was the highest in CK treatment; The grain fat content of LN treatment increased by 1.7%-2.0% compared with CK treatment, and the isoflavone content of HN treatment decreased by 5.2%-11.2% compared with CK treatment. The structural equation model indicated that N application negatively regulated fat content and indirectly affected soybean yield; Negatively regulate the number of nodules and indirectly affect the content of isoflavones in soybean. In general, the N application at V2 stage was more beneficial to soybean yield and nitrogen fixation, while the N application at R1 stage was more beneficial to soybean grain fat content. The reasonable amount of N application should be controlled at 5 mg N/kg soil.
nitrogen fertilizer / nodule / nitrogenase activity / yield / fat / isoflavone {{custom_keyword}} /
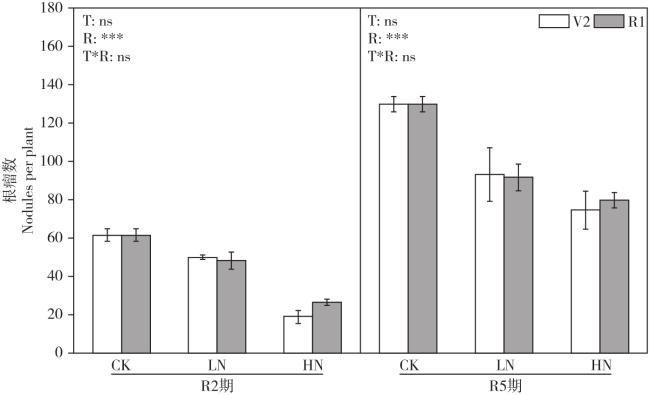
Fig. 1 Effects of N fertilizer application time and rate on soybean nodule number图1 氮肥施用时期和施用量对大豆根瘤数量的影响 |
Table 1 Effects of N application time and rate on on grain yield, fat and isoflavone content of soybean表1 氮肥施用时期和施用量对大豆籽粒产量、脂肪和异黄酮含量的影响 |
| 施氮时期 Time | 施氮量 Rate | 产量 Yield /(g/pot) | 脂肪含量 Fat /% | 异黄酮含量 Isoflavone content /(μg/g) |
|---|---|---|---|---|
| CK | 19.31 | 22.42 | 658.05 | |
| V2期 V2 stage | LN | 25.36 | 22.81 | 595.53 |
| HN | 22.56 | 22.66 | 584.04 | |
| R1期 R1 stage | LN | 23.95 | 22.87 | 654.67 |
| HN | 19.87 | 22.71 | 623.87 | |
| 方差分析Analysis of variance | ||||
| 施N时期Time | ns | ns | ns | |
| 施N量Rate | *** | ** | ** | |
| 施N时期*施N量Time*Rate | ns | ns | ns | |
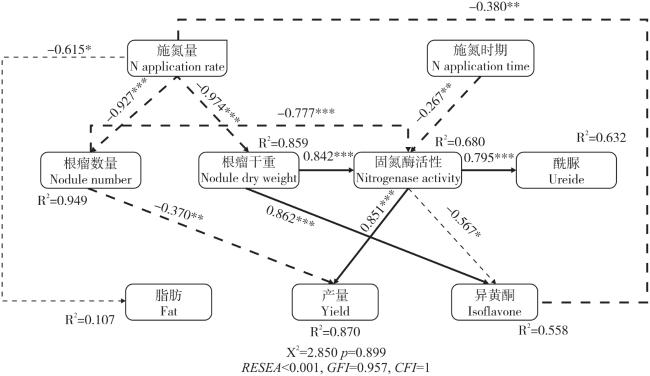
Fig. 5 SEM of nodule characteristics, nitrogenase activity, ureide, yield, fat and isoflavone with various N application time and rate图5 施氮量和施氮时期下根瘤特性、固氮酶活性、酰脲、籽粒产量、脂肪及异黄酮含量之间的结构方程图 |
| 1 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 2 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 3 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 4 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 5 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 6 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 7 |
严君, 韩晓增, 王守宇, 等. 不同施氮量及供氮方式对大豆根瘤生长及固氮的影响[J]. 江苏农业学报, 2010, 26(1): 75-79. DOI: 10.3969/j.issn.1000-4440.2010.01.015 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 8 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 9 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 10 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 11 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 12 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 13 |
郭泰, 刘秀芝, 郑殿峰, 等. 氮素后移施肥对大豆产量及品质的影响[J]. 大豆科学, 2015, 34(1): 168-171. DOI: 10.11861/j.issn.1000-9841.2015.01.0168 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 14 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 15 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 16 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 17 |
冯丽娟, 朱洪德, 于洪久. 栽培措施对高油大豆产量及品质性状的影响[J]. 中国油料作物学报, 2008, 30(2): 206-211. DOI: 10.3321/j.issn: 1007-9084.2008.02.014 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 18 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 19 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 20 |
张志良. 植物生理学实验指导[M]. 2版. 北京: 高等教育出版社, 1990.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 21 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 22 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 23 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 24 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 25 |
张洪刚, 周琴, 何小红, 等. 播期、密度和肥料对菜用大豆南农9610产量和品质的影响[J]. 江苏农业学报, 2008, 24(5): 662-667. DOI: 10.3969/j.issn.1000-4440.2008.05.023 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 26 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 27 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 28 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 29 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 30 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 31 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 32 |
赵双进, 张孟臣, 杨春燕, 等. 栽培因子对大豆生长发育及群体产量的影响 Ⅱ.肥水、生长调控措施对产量的影响[J]. 中国油料作物学报, 2003, 25(2): 48-51. DOI: 10.3321/j.issn: 1007-9084.2003.02.013 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 33 |
高阳, 傅积海, 章建新, 等. 施氮量对高产春大豆光合特性及产量的影响[J]. 中国农学通报, 2020, 36(14): 34-40. DOI: CNKI:SUN:ZNTB.0.2020-14-007 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 34 |
姜璐, 宁海龙, 李文霞, 等. 氮肥施用量对超早熟大豆源库关系、产量和品质的影响[J]. 中国农学通报, 2013, 29(30): 105-111. DOI: 10.3969/j.issn.1000-6850.2013.30.020 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 35 |
甘银波, 陈静. 大豆不同生长阶段施用氮肥对生长、结瘤及产量的影响[J]. 大豆科学, 1997, 16(2): 125-130. DOI: CNKI:SUN:DDKX.0.1997-02-005 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 36 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 37 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 38 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 39 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 40 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 41 |
冯博政, 刘晓静, 郝凤, 等. 外源氮对紫花苜蓿固氮酶活性和酰脲含量的影响及其相关关系研究[J]. 草地学报, 2016, 24(2): 351-357. DOI: 10.11733/j.issn.1007-0435.2016.02.016 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 42 |
田艳洪, 刘元英, 张文钊, 等. 不同时期施用氮肥对大豆根瘤固氮酶活性及产量的影响[J]. 东北农业大学学报, 2008, 39(5): 15-19. DOI: 10.19720/j.cnki.issn.1005-9369.2008.05.004 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 43 |
严君, 韩晓增. 盆栽条件下土壤无机氮浓度对大豆结瘤、固氮和产量的影响[J]. 中国农业科学, 2014, 47(10): 1929-1938. DOI: 10.3864/j.issn.0578-1752.2014.10.006 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 44 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 45 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 46 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 47 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 48 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 49 |
房春红, 陈秀双, 刘杰, 等. 大豆固氮酶活性与酰脲含量的关系[J]. 东北农业大学学报, 2008, 39(3): 9-12. DOI: 10.19720/j.cnki.issn.1005-9369.2008.03.003 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 50 |
刘晓玲, 杜文华, 宋超. 氮磷肥施用量对红三叶中异黄酮含量的影响[J]. 西北农业学报, 2010, 19(7): 159-163, 180. DOI: 10.3969/j.issn.1004-1389.2010.07.035 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 51 |
童长春, 刘晓静, 吴勇, 等. 内源异黄酮对紫花苜蓿结瘤固氮及氮效率的调控研究[J]. 草业学报, 2022, 31(3): 124-135. DOI: 10.11686/cyxb2021011 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 52 |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 53 |
李奇真, 孙克用, 卢增辉, 等. 夏大豆施肥生理基础及高产栽培技术研究[J]. 中国农业科学, 1989, 22(4): 41-48. DOI:CNKI:SUN:ZNYK.0.1989-04-007 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| 54 |
柯丹霞, 徐勤朕, 杨娜, 等. 高氮抑制豆科植物结瘤固氮机制研究进展[J]. 生物技术通报, 2019, 35(10): 40-45. DOI: 10.13560/j.cnki.biotech.bull.1985.2019-0657 .
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
 PDF(3387 KB)
PDF(3387 KB)
 Fig. 1 Effects of N fertilizer application time and rate on soybean nodule number
Fig. 1 Effects of N fertilizer application time and rate on soybean nodule number Fig. 2 Effects of N fertilizer application time and rate on soybean nodule dry weight
Fig. 2 Effects of N fertilizer application time and rate on soybean nodule dry weight Fig. 3 Effects of N fertilizer application time and rate on nitrogenase activity of soybean nodule
Fig. 3 Effects of N fertilizer application time and rate on nitrogenase activity of soybean nodule Fig. 4 Effects of N fertilizer application time and rate on ureide content of soybean nodule
Fig. 4 Effects of N fertilizer application time and rate on ureide content of soybean nodule Table 1 Effects of N application time and rate on on grain yield, fat and isoflavone content of soybean
Table 1 Effects of N application time and rate on on grain yield, fat and isoflavone content of soybean Fig. 5 SEM of nodule characteristics, nitrogenase activity, ureide, yield, fat and isoflavone with various N application time and rate
Fig. 5 SEM of nodule characteristics, nitrogenase activity, ureide, yield, fat and isoflavone with various N application time and rate/
| 〈 |
|
〉 |